Is there a catalytic effect of HBr on the bromination of aromatics?
Recently, much attention has been paid to the theoretical study of the catalytic
action of HCl in the chlorination of aromatics.
Galabov, B.; Koleva, G.; Kong, J.; Schaefer, H. F., III; Schleyer, P. V. R.
Eur. J. Org. Chem. 2014, 2014, 6918−6924.
DOI: 10.1002/ejoc.201402765
T.D. Marforio, A. Bottoni, P. Giacinto, F. Zerbetto
J. Phys. Chem. C 2017, 121, 27674−2768
DOI: 10.1021/acs.jpcc.7b07296
Van Lommel, R.; Moors, S. L.; De Proft, F.
Chem. - Eur. J. 2018, 24, 7044−7050.
DOI: 10.1002/chem.201800385
T. Stuyver, D. Danovich, F. De Proft, and S. Shaik.
J. Am. Chem. Soc. 2019, 141, 9719−9730.
DOI: 10.1021/jacs.9b04982
What about the catalytic action of HBr in bromination reactions?
Experimental data
AV Shernyukov, AM Genaev, GE Salnikov, HS Rzepa, VG Shubin.
Noncatalytic bromination of benzene: A combined computational and experimental study.
J. Comput. Chem. 2016, 37 (2), 210-225.
DOI: 10.1002/jcc.23985
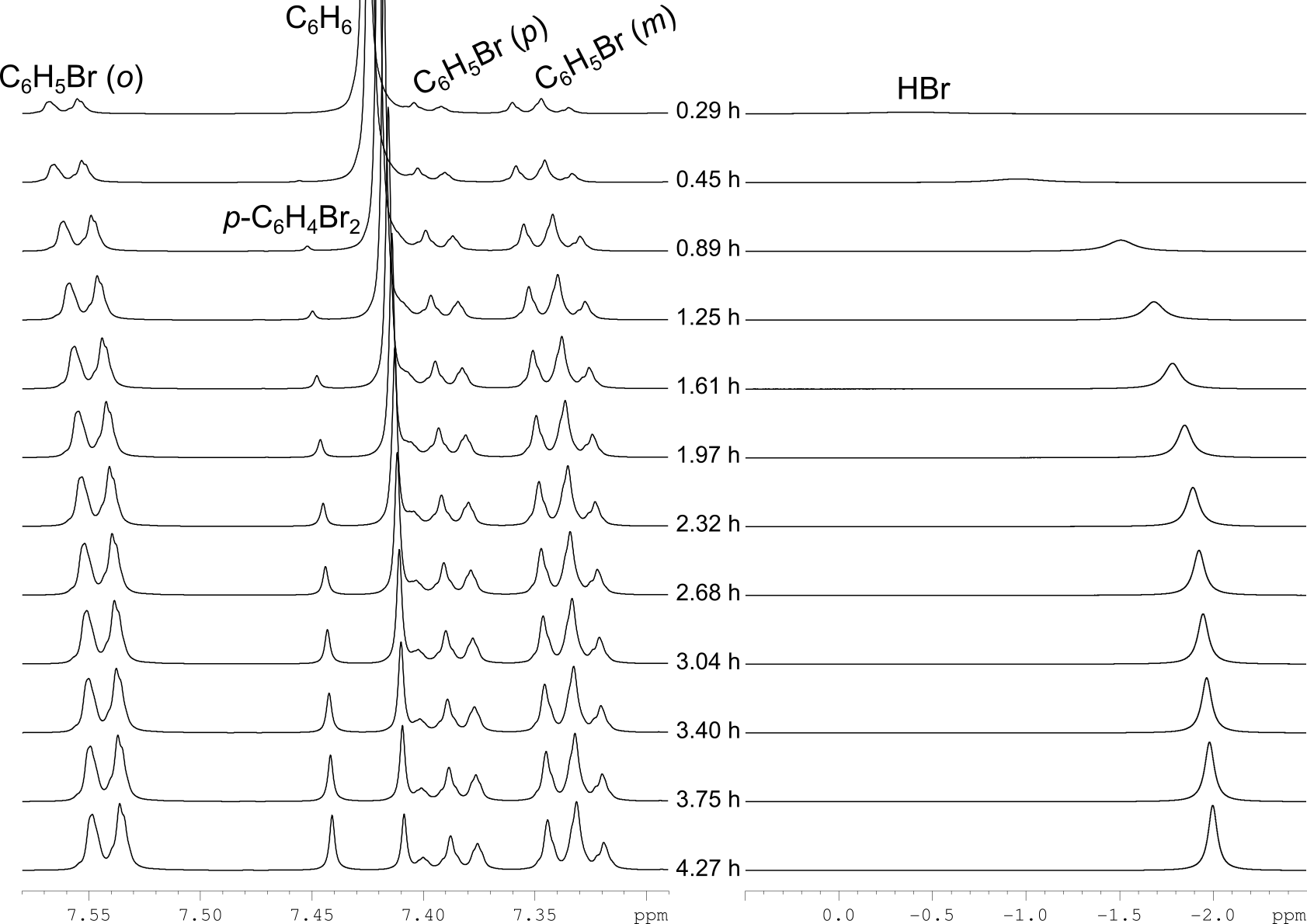
C6H6 + Br2 → C6H5Br + C6H4Br2 + HBr
(0.032 ml of benzene, 0.37 ml of bromine, 0.11 ml of CD2Cl2 in sealed NMR tube at 0 °C)
1H NMR (600 MHz) monitoring
 |
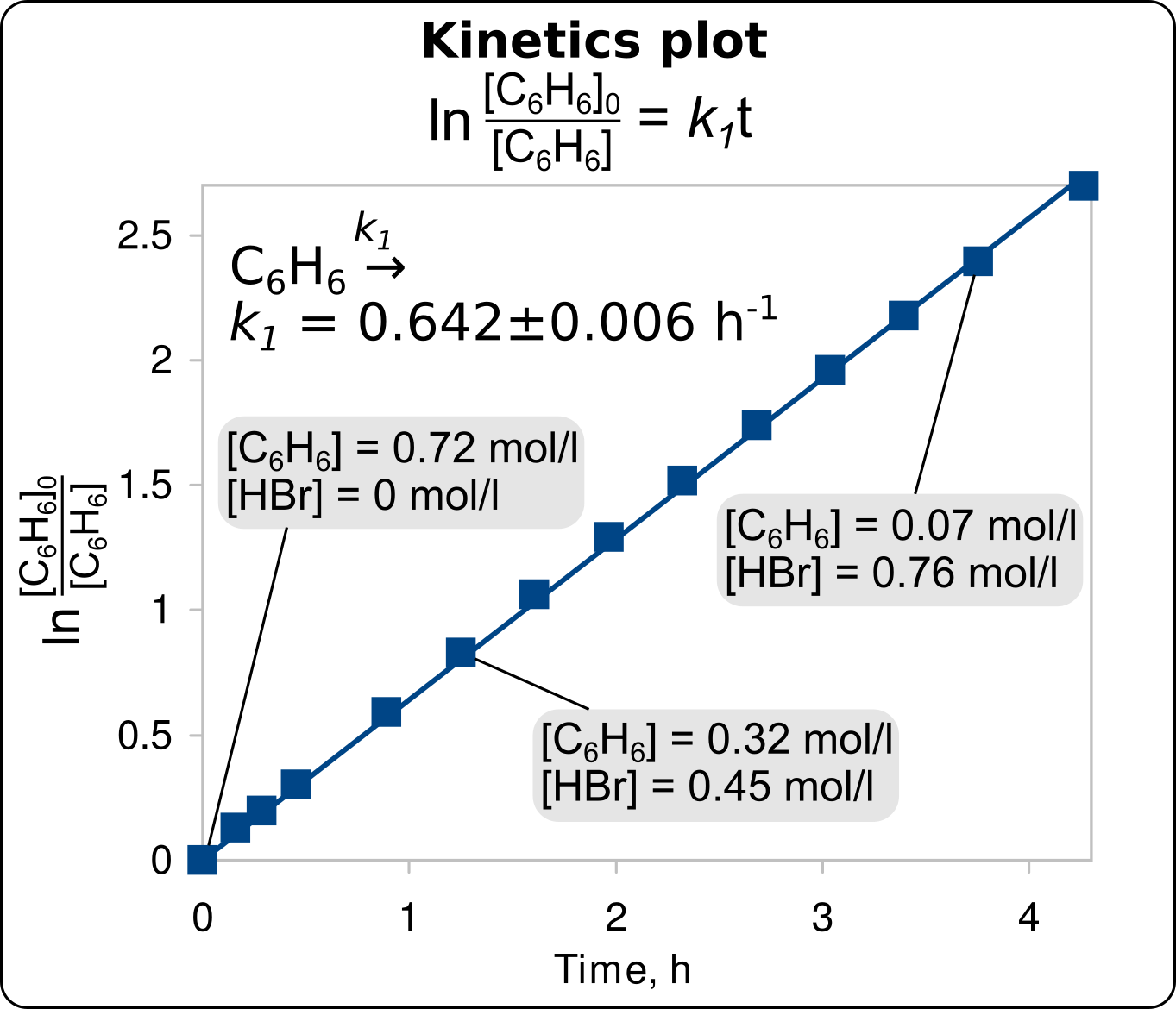 |
According to these kinetic data, the hydrogen bromide formed during the reaction has no effect on the rate constant of benzene bromination.
The catalytic effect of HBr is absent.
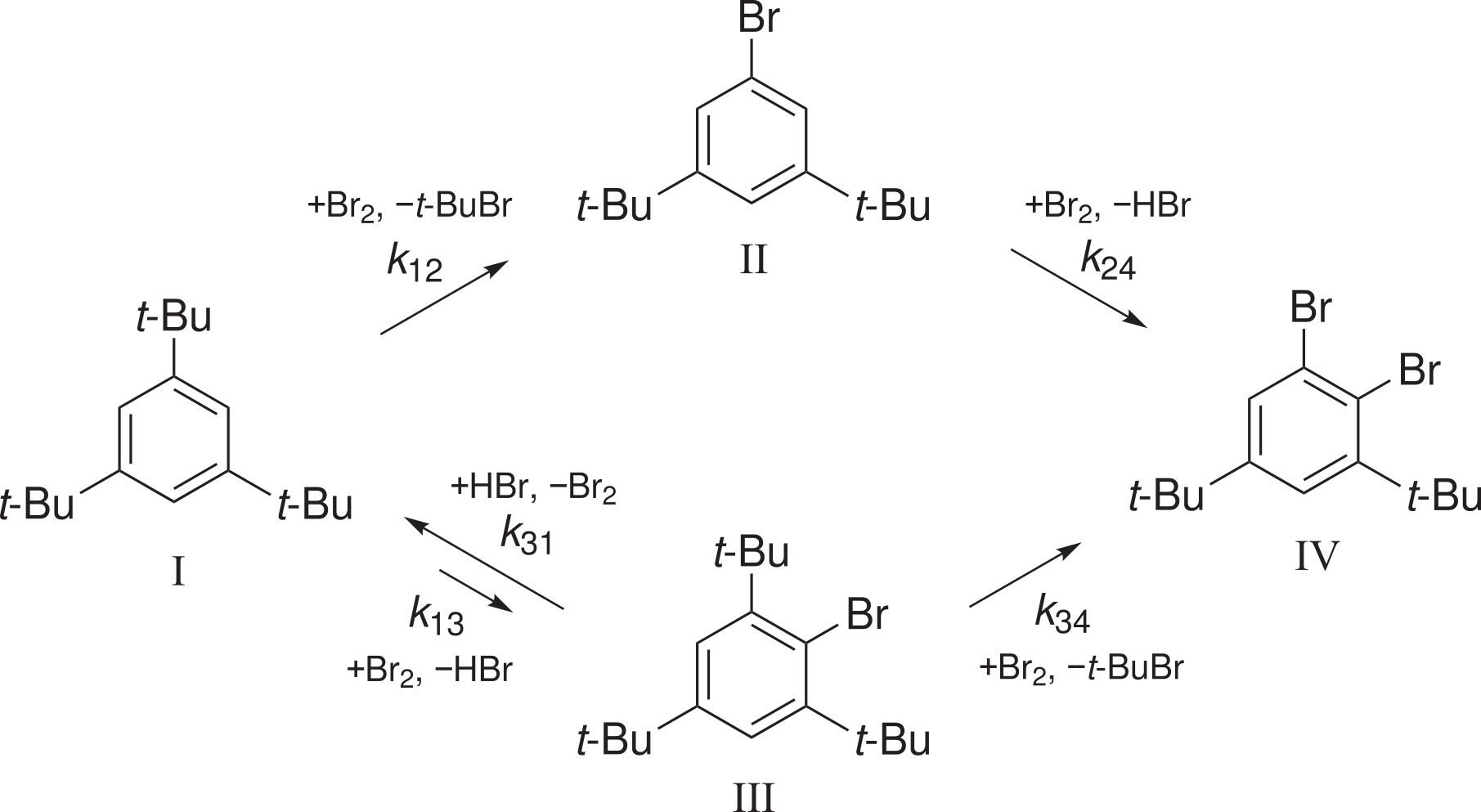
A Genaev, HS Rzepa, AV Shernyukov, G Salnikov, V Shubin.
Elevated Reaction Order of 1,3,5-tri-tert-butylbenzene Bromination as Evidence of a Clustered Polybromide Transition State: a Combined Kinetic and Computational Study.
Org. Biomol. Chem., 2019, 17, 3781-3789.
DOI: 10.1039/C9OB00607A
Scheme of the reaction
 |
Kinetics plot
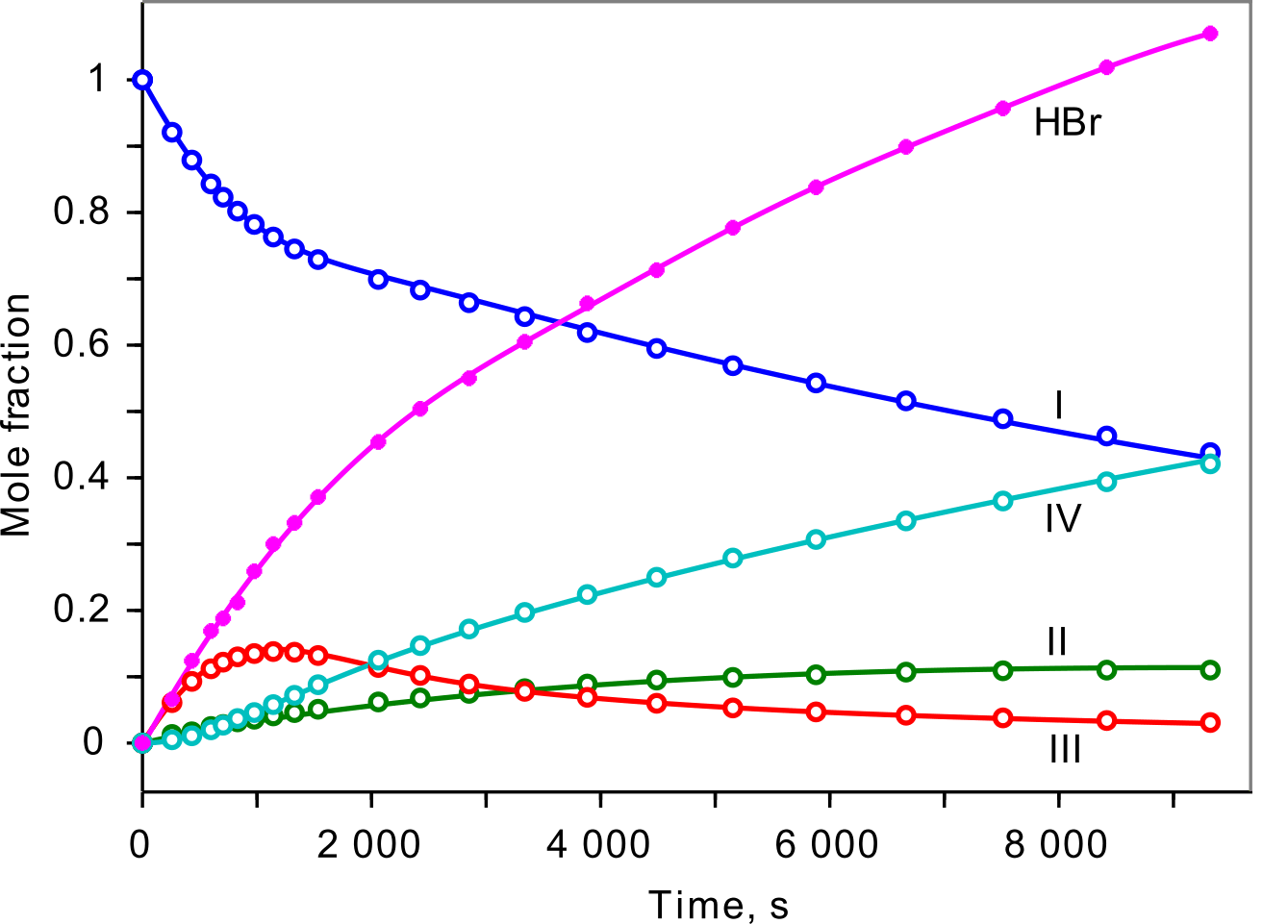 |
It has been found that hydrogen bromide affects the rate of the bimolecular debromination reaction III+HBr→I+Br2,
but has no effect on the rate constants of the bromination reactions I→III and II→IV. There is no catalytic effect of HBr.
DFT calculations
Geometry optimization by DFT/PBE/Λ1 (PRIRODA program [Chem. Phys. Lett. 1997, 281, 151; Russ. Chem. Bull. 2005, 54, 820])
with thermochemical ΔG (298.15 K) and
Grimme's D3(BJ)
dispersion corrections
[J.Chem. Phys. 2010, 132, 154104;
J. Comp. Chem. 2011, 32, 1456]
for the energy of optimized geometry
Basis Λ1
[Chem. Phys. Lett. 2005, 416, 116;
Theor Chem Acc (2019) 138: 40]:
H (6s,2p)→[2s,1p]; C (10s,7p,3d)→[3s,2p,1d]; Br (19s,15p,11d)→[5s,4p,2d]
| On figure(s) below click on the level title to download xyz file |
==> |
| Click on energy level to view 3D structure in browser (run JSmol) |
==> |
| 
|
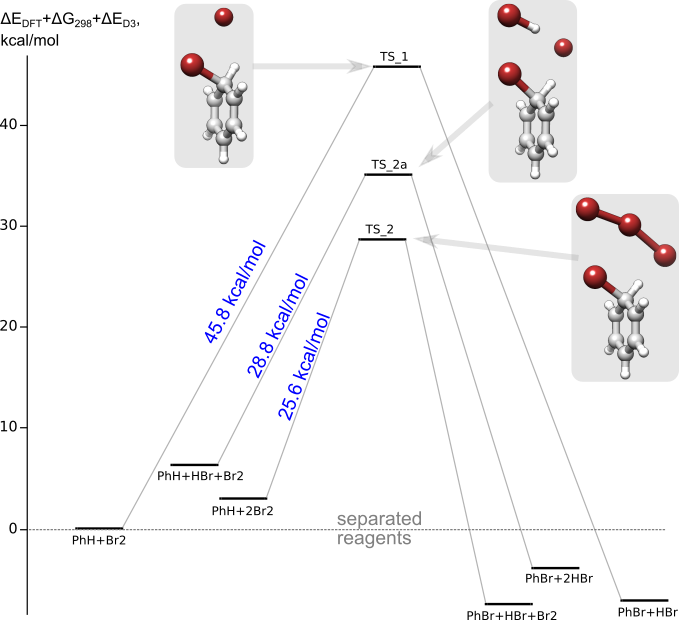
Calculations show that the addition of HBr to the PhH+Br2 system leads to a significant decrease in the barrier of the bromination reaction.
But if we involve in the consideration the trimolecular reaction PhH+Br2+HBr, then we cannot ignore another trimolecular reaction, PhH+Br2+Br2.
Under conditions of excess bromine, the statistical probability of the formation of a pre-reaction complex comprising two Br2 molecules is higher than that of Br2+HBr.
And the reaction barrier is lower. Therefore, the catalytic effect of HBr should not manifest itself. As observed in the experiment.
03.07.2019
| |
|
| |
|