Новосибирский институт органической химии им. Н.Н. Ворожцова СО РАН
Лаборатория изучения механизмов органических реакций
 |
 |
 |
 |
 |
 |
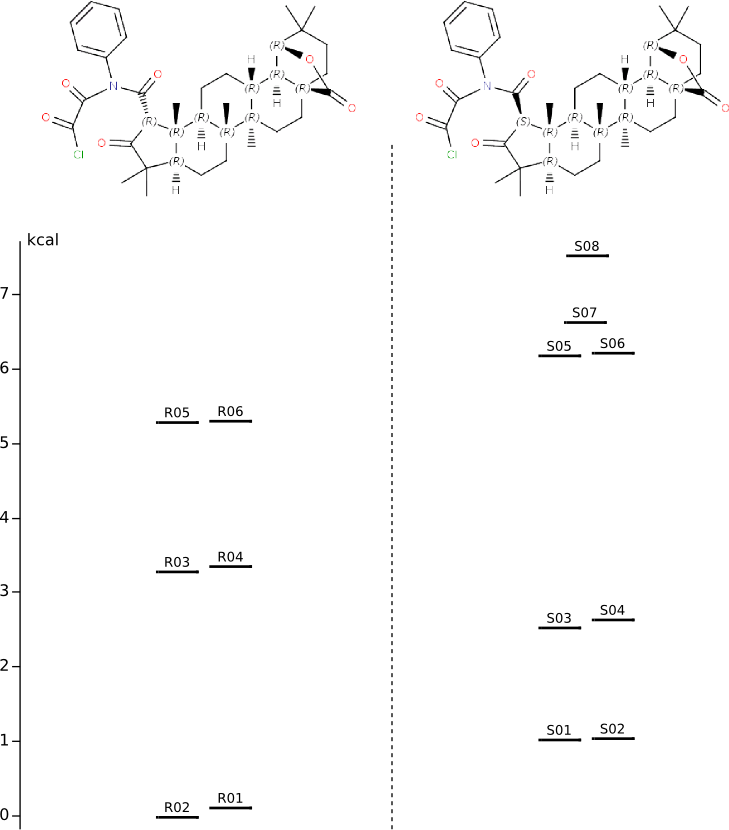
Conformational anlysis of R- and S-intermediates
Methods of conformational anlysis: MM (Vconf) — MOPAC (RM1) — DFT (PRIRODA).Energy (without ZPE) by DFT/PBE/L22 (cc-pCVTZ) for DFT/PBE/L1 (cc-pVDZ) geometry (PRIRODA program).

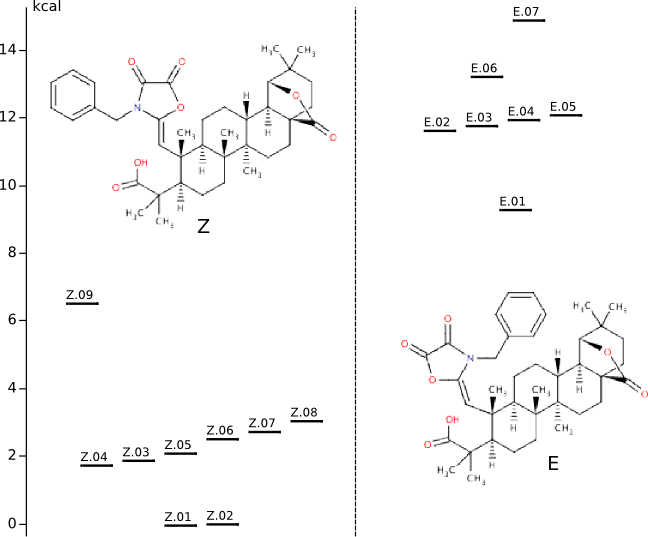
Conformational anlysis of Z and E
Methods of conformational anlysis: MM (Vconf) — MOPAC (RM1) — DFT (PRIRODA).Energy (without ZPE) by DFT/PBE/L22 (cc-pCVTZ) for DFT/PBE/L1 (cc-pVDZ) geometry (PRIRODA program).
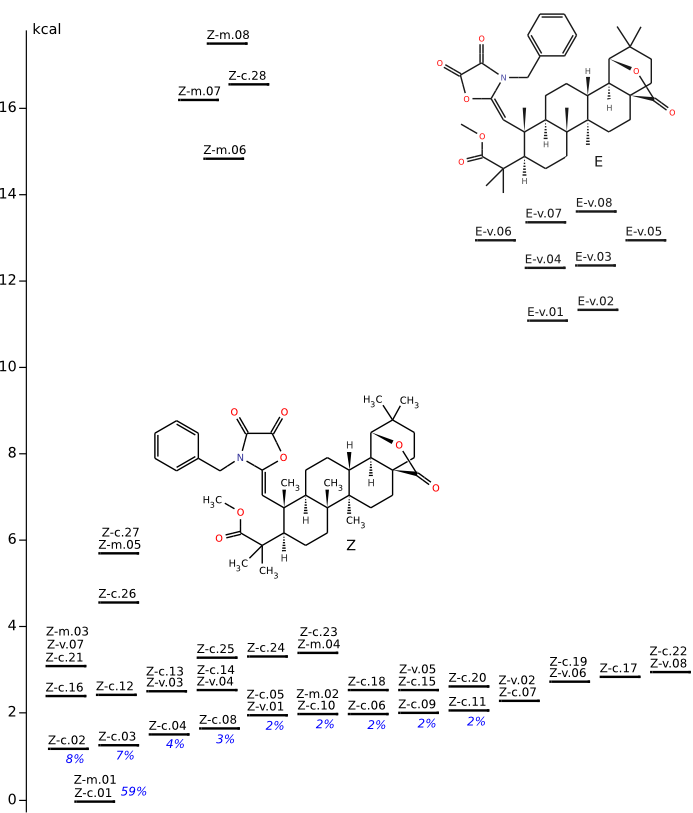
Conformational anlysis of Z and E
Methods of conformational anlysis: MM (marvin (for Z only) + Vconf) — MOPAC (RM1) — DFT (PRIRODA).Energy (without ZPE) by DFT/PBE/L22 (cc-pCVTZ) for DFT/PBE/L1 (cc-pVDZ) geometry (PRIRODA program).
Conformational anlysis and PES of lactone L
Methods of conformational anlysis: MM (Vconf) — MOPAC (RM1) — DFT (PRIRODA).PES investigation by DFT/PBE/L1 (cc-pVDZ) (PRIRODA program).
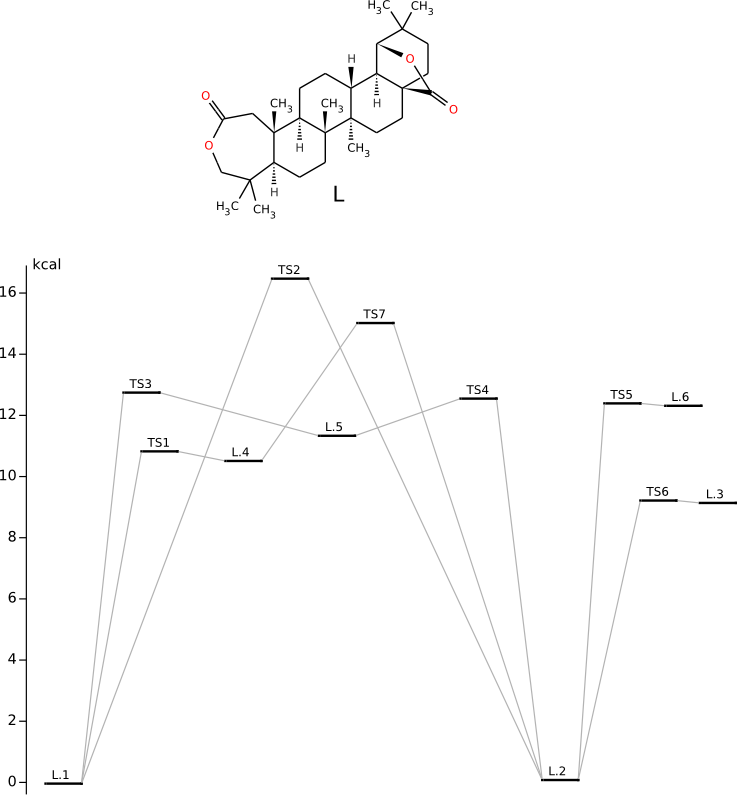
IRC path for L.1-TS3-L.5-TS4-L.2 reaction
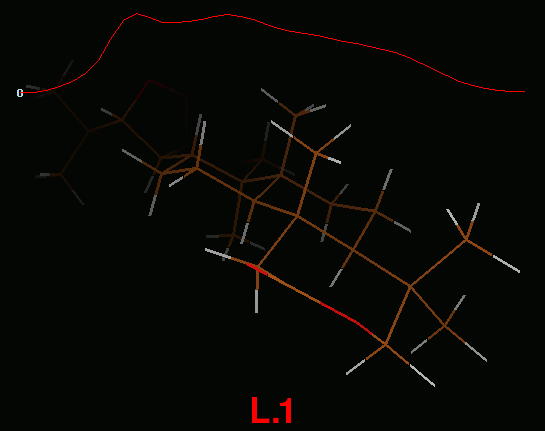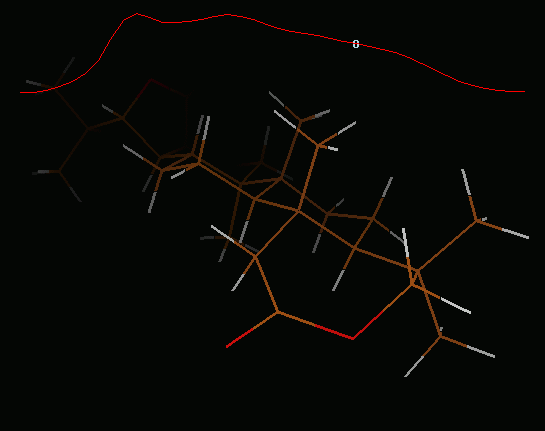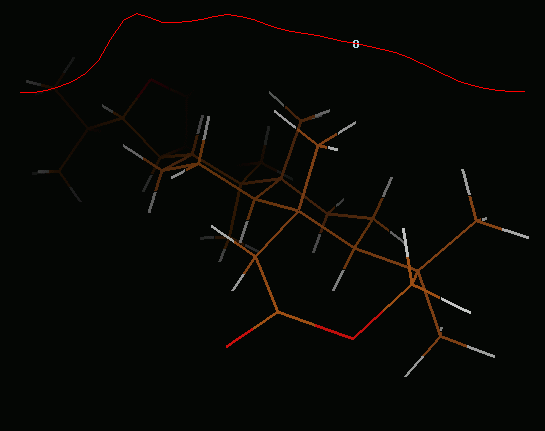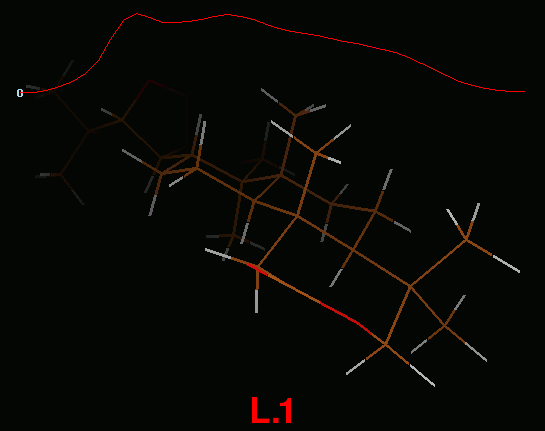
IRC path (concatenated xyz) for L.1-TS3-L.5-TS4-L.2 reaction
xyz-files with calculated chemical shifts (DFT/PBE/L22 (cc-pCVTZ, Priroda)) of most stable conformers:
L.1.xyzppm L.2.xyzppm
Conformational anlysis of 2Z- and 2E- methyl 5-[3-[(4-methoxyphenyl)methyl]-4,5- dioxo-1,3-oxazolidin-2-ylidene]pentanoate
Methods of conformational anlysis: MM (Vconf) — DFT (PRIRODA).Energy (without ZPE) by DFT/PBE/L22 (cc-pCVTZ) for DFT/PBE/L1 (cc-pVDZ) geometry (PRIRODA program).

xyz-files with calculated chemical shifts averaged by all conformers (by Boltzmann distribution):
Z.xyzppm E.xyzppm