Новосибирский институт органической химии им. Н.Н. Ворожцова СО РАН
Лаборатория изучения механизмов органических реакций
 |
 |
 |
 |
 |
 |
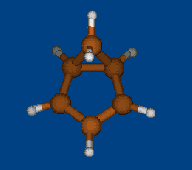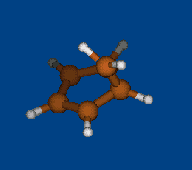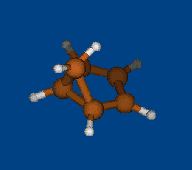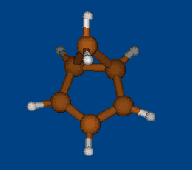
Conformers of tris(2,2,6,6-tetramethylbenzo[1,2-d;4,5-d']bis[1,3]dithiol-4-yl)methyl cation and transition states of "two-ring flip" aryl rotations
Energy (without ZPE) and geometry optimization by DFT/PBE/L1 (cc-pVDZ) (PRIRODA program).
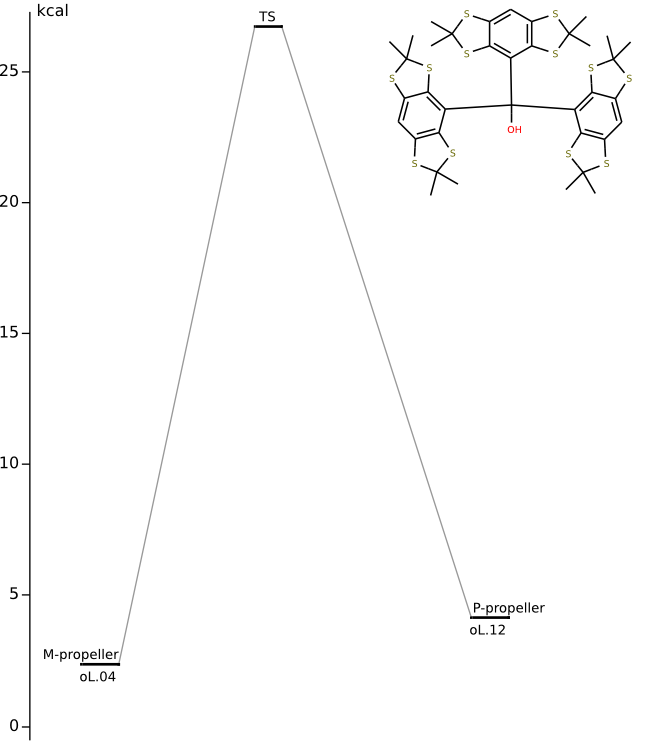
Energy levels and calculated chemical shifts
Energy (without ZPE) and chemical shifts by DFT/PBE/L22 (cc-cpVTZ) for DFT/PBE/L1 (cc-pVDZ) geometry (PRIRODA program).
| 
|

Chemical shifts averaged by conformers
Protonation of sulphur atoms and disclosure of dithiole ring in conformer "a"

Energy (without ZPE) and chemical shifts by DFT/PBE/L22 (cc-cpVTZ) for DFT/PBE/L1 (cc-pVDZ) geometry (PRIRODA program).
| 
|

© Журнал структурной химии
© Journal of Structural Chemistry