| On figure(s) below click on the level title to download xyz file | ==> |
| Click on energy level to view 3D structure in browser (run JSmol) | ==> |

| 
|


| 
|



| 
|

| A | A-B | B | B-B | C | C-A | ref | |
 |
0.00
0.00a |
16.37
17.43a |
1.96
7.04a |
4.37
3.74a |
12.64
12.41a |
27.19
26.96a |
A. Greer, J. Am. Chem. Soc. 2001, 123, 10379-10386 Calculated (B3LYP/6-31G*): 7(A) 0.00, 16(B) 4.86, TS15(A-B) 16.95, 21(C) 12.94, TS20(C-A) 26.58 kcal/mol. The author believes that the chair-chair interconversions of pentathiepin 7(A) passes through TS20(C-A). The lower energy path through TS15(A-B) is not considered. B.L. Chenard, D.A. Dixon, R.L. Harlow, D.C. Roe, T. Fukunaga, J. Org. Chem. 1987, 52, 2411-2420 Calculated (MP2): 6-C(A) 0.00, 6-TB(B-B) 4.1, 6-B(C) 13.1, 6-TS(1)(C-A) 29.2 kcal/mol. Chair-chair interconversion proceeds via TS(1). |
 |
0.71
0.00 |
20.40
19.01 |
3.39
3.21 3.47 |
6.17 | 12.20 | 25.76 | E.M. Brzostowska, M. Paulynice, R. Bentley, A. Greer Planar Chirality due to a Polysulfur Ring in Natural Pentathiepin Cytotoxins. Implications of Planar Chirality for Enantiospecific Biosynthesis and Toxicity Chem. Res. Toxicol. 2007, 20, 1046-1052 Calculated by B3LYP/6-31G(d): 3a(A) 0.00, TS-1(C-A) 24.7, 3b(B) 5.9, TS-2 19.0, 3c(A') -0.3 kcal/mol. Mechanism 3a⇄TS-1⇄3b⇄TS-2⇄3c is proposed with barrier 24.7 kcal/mol. |
 |
0.00 | 19.12 | 4.16 | 6.47 | 10.83 | 27.54 | |
 |
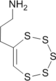
0.00 | 19.68 | 3.72 | 6.18 | 11.18 | 27.35 | Ando W., Kumamoto Y., Tokitoh N., Tetrahedron Letters, 28(41), 4833–4836 (1987) 1H NMR 1.26(s,6H), 1.57(s,6H): barrier > 16 kcal/mol |
 |
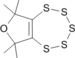
0.00 | 29.34 | 6.33 | 9.68 | 10.97 | 23.11 | Tokitoh N., Ishizuka H., Ando W., Chem. Lett. 1988, 657-660 1H NMR 1.46(s,6H), 1.74(s,6H): barrier > 16 kcal/mol |
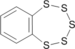 |
0.00
0.00a |
23.58
27.32a |
5.48
9.91a |
7.33
6.96a |
12.55
11.26a |
25.83
26.59a |
|
 |
0.00 | 23.76 | 6.49 | 7.78 | 10.98 | 27.52 | |
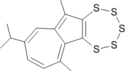 |
0.00 | 23.72 | 5.08 | 9.17 | 25.39 | O. Sato, T. Saito, M. Iwase, A. Sakai, Heterocycles 2016, 93, 714-722 No data about barrier |
|
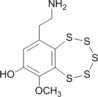 |
0.68
0.00 |
28.38
27.63 |
7.82
6.75 |
9.33
9.45 |
11.18
10.96 |
23.72
24.74 |
P.A. Searle, T.F. Molinski, J. Org. Chem. 1994, 59, 6600-6605 Lissoclinotoxin A is chiral: protons of CH2 group are diastereotopic on the NMR time scale. |
 |
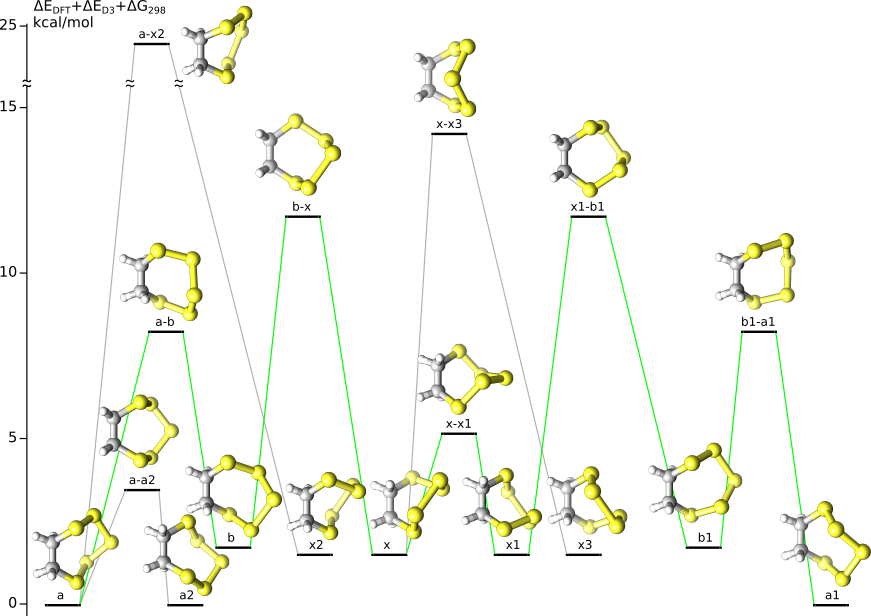
B.S. Davidson, P.W. Ford, M. Wablman,
"Chirality in unsymmetrically substituted benzopentathiepins: The result of a high barrier to ring inversion",
Tetrahedron Lett. 1994, 35 (39), 7185-7188 R = H (varacin): according to MM calculations (CVFF force field) half-chair conformation 1c(C-A) is higher TS of the ring inversion (34 kcal/mol) R = COOCH2CH2Si(CH3)3: diastereotopic ArCH2 signals do not broaden at 100 °C (barrier > 21 kcal/mol) R = (S)-CONHCHMe(1-naphthyl): slow interconversion of diastereomers at room temperature over a matter of days in deuteriochloroform (barrier ~25 kcal/mol) |
||||||
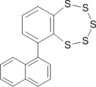 |
0.07
0.00 |
26.06
25.64 |
5.58
5.70 |
7.93
7.67 |
11.90
11.96 |
25.23
25.70 |
R. Sato, H. Ohta, T. Yamamoto, Sh. Nakajo, S. Ogawa, A. Alam Synthesis of novel axially chiral cyclic benzopolysulfides Tetrahedron Letters 48 (2007) 4991–4994 Experimental ∆G298≠ is 24.3 kcal/mol. |
 |
0.11
0.00 |
27.57
26.87 |
6.64
6.17 |
8.23
9.11 |
11.34
11.54 |
26.35
24.51 |
B.L. Chenard, D.A. Dixon, R.L. Harlow, D.C. Roe, T. Fukunaga, J. Org. Chem. 1987, 52, 2411-2420 Experimental barrier 19.3 kcal/mol (ref. 17, unconfirmed data; additionally, CH2 is singlet, not AB system [J. Am. Chem. Soc. 1985, 107, 3871-3879]) |
 |
0.00
0.22 |
28.61
29.76 |
7.44
7.46 |
9.70
10.82 |
10.08
10.15 |
23.74
23.82 |
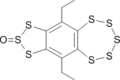
T. Kimura, M. Hanzawa, K. Tsujimura, T. Takahashi, Y. Kawai, E. Horn, T. Fujii, S. Ogawa, R. Sato Preparation and Conformational Analysis of 6,10-Diethyl[1,2,3]trithiolo[4,5-h]benzopentathiepin Monoxides: Isolation and Optical Properties of Chiral Benzopentathiepin Derivatives Bull. Chem. Soc. Jpn., 75, 817–824 (2002) Experimental ∆G298≠ is 23.9 kcal/mol. |
 |
0.12
0.00 |
26.87
26.86 |
7.37
7.39 |
8.75
8.84 |
13.80
14.05 |
21.93
21.87 |
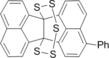
Y. Sugihara, H. Takeda, J. Nakayama Synthesis of Pentathiepanes and Isolation of the Conformers Based on High Inversion Barrier of the Pentathiepane Ring Eur. J. Org. Chem. 1999, 597-605; Tetrahedron Letters 39 (1998) 2605-2608 Experimental ∆G298≠ is 24.0 kcal/mol. Mechanism A⇄C⇄24⇄C'⇄A' is proposed, A⇄C stage being limited. |
 |
0.00 | 30.46 | 6.47 | 8.51 | 13.50 | 16.92 | |
 |
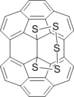
0.00 | 31.81 | 7.57 | 10.17 | 12.91 | 17.66 | M. Anafcheh, F. Ektefa Cyclosulfurization of C60 and C70 fullerenes: a DFT study Struct Chem (2015) 26:1115–1124 Sheer confusion. Calculated barrier 37 kcal/mol, but depicted in fig. 4 TS is B-B. Low energy TS C-A is not mentioned. |
| A | A-B | B | B-B | C | C-A | ref |