Новосибирский институт органической химии им. Н.Н. Ворожцова СО РАН
Лаборатория изучения механизмов органических реакций
 |
 |
 |
 |
 |
 |
Ring current in arenonium ions. Hyperconjugation?
See responses to Henry Rzepa post.About ring current in arenonium ions: V.A. Koptyug, Bulletin of the Academy of Sciences of the USSR, Division of chemical science May 1974, Volume 23, Issue 5, pp 1031-1045 DOI
"The transition of the protonated carbon atom to a hybridization state of the sp3 type should be accompanied by the substrate molecule losing its aromaticity. However, it is curious that evidently a high anisotropy of the magnetic susceptibility is retained for the protonated benzene ring, which is characteristic for aromatic systems and is usually explained by the induction of a ring pi-electron current in them."
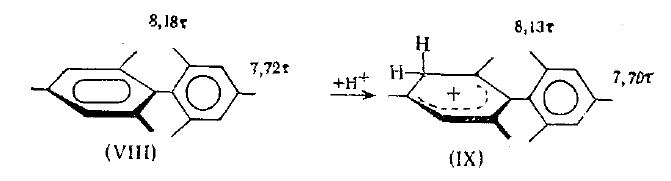
"... The reasons for this phenomenon are not entirely clear. One of them is evidently the effective participation of the C-H bonds of the ring,"aliphatic" fragments of the benzenonium ions in the sigma,pi-conjugation with the charged pentadienyl portion of the ion, as a result of which a complete interruption of the ring conjugation chain fails to occur during protonation."
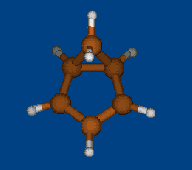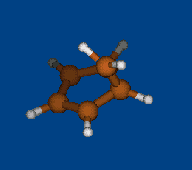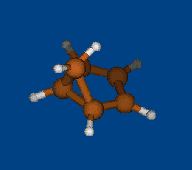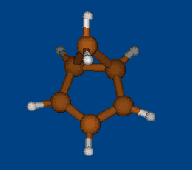
Replace CH2 with CMe2 in carbocation (IX) should decrease hyperconjugation.
Geometry optimization by DFT/PBE/L1 (PRIRODA program, basis aka cc-pVDZ)
Chemical shifts and NICS calculations by DFT/PBE/L22 (PRIRODA program, basis aka cc-pCVTZ)
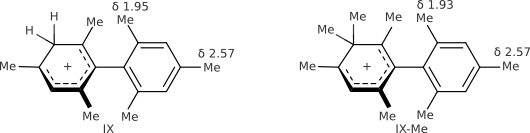
There is no effect on anizotropy.
Click on links below to run java-applet jmol. Check "show chemical shifts" to see calculated shieldings and NICS.
IX IX-Me