Новосибирский институт органической химии им. Н.Н. Ворожцова СО РАН
Лаборатория изучения механизмов органических реакций
 |
 |
 |
 |
 |
 |
Rzepa's semibullvalene
Henry Rzepa has found intriguing noncharged nonclassical structure: 1,5-dimethyl-2,6-diazatricyclo[3.3.0.02,8]octa- 3,6-diene-4,8-dicarbonitrile. Is this structure bishomoaromatic? There is NICS data.Energy (without ZPE) and NMR chemical shifts by DFT/PBE/L22 (cc-pCVTZ) (PRIRODA program) for DFT/PBE/L1 (cc-pVDZ) geometry.
| 
|
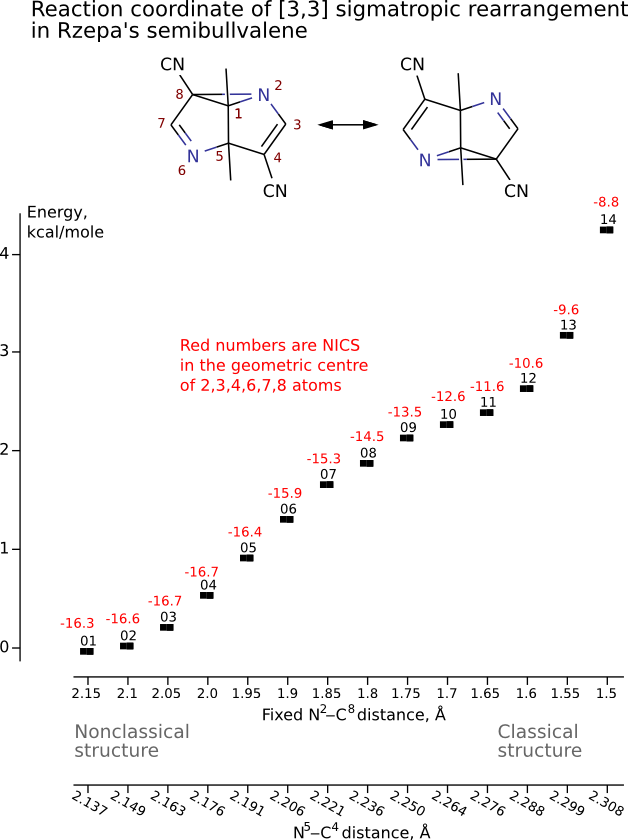 Not N5-C4 but N6-C4
Not N5-C4 but N6-C4
NICS of the nonclassic structure (-16.3 ppm) is more negative than classic one (about -10 ppm) or than the structure with two cycloprorane rings (-9.9 ppm). (In the classic structures negative NICS is due to sigma aromaticity of cycloprorane.)
Thus, NICS data support bishomoaromaticity of the nonclassic structure.